WASTE & FUELS OF THE FUTURE
How can we transition to a net-zero green economy? How do we create energy resources from waste materials? And how can these energy resources reinforce the circular economy?
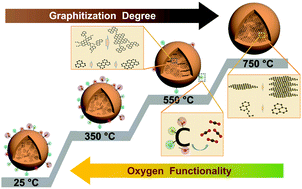
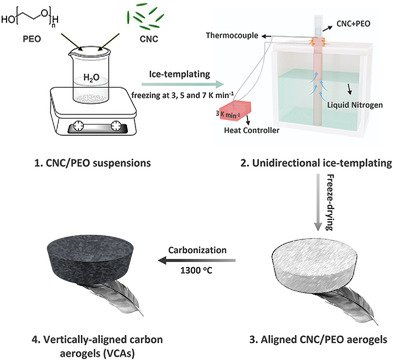
Titirici group’s ambition is to recycle bio and plastic waste into advanced materials which are components of future batteries and fuel cells.
Professor Magda Titirici discussed what the sustainable solutions are for the next generation of energy storage and conversion technologies. It was exciting to see our research through the eyes of some very talented young artists and advocating for the urgency of developing and implementing sustainable technologies to save the planet.